News!
We're thrilled to announce that our groundbreaking research has made it to the COVER PAGE of the Journal of Natural Products (June 23, 2023 Volume 86, Issue 6)!
Nicolaioidesin C, a remarkable natural product from Bosenbergia pandurata , holds immense promise as an antitumor agent targeting pancreatic cancer. Read the cover story in Journal of Natural Products:
https://pubs.acs.org/toc/jnprdf/86/6
Nicolaioidesin C, a remarkable natural product from Bosenbergia pandurata , holds immense promise as an antitumor agent targeting pancreatic cancer. Read the cover story in Journal of Natural Products:
https://pubs.acs.org/toc/jnprdf/86/6
Our latest Publication
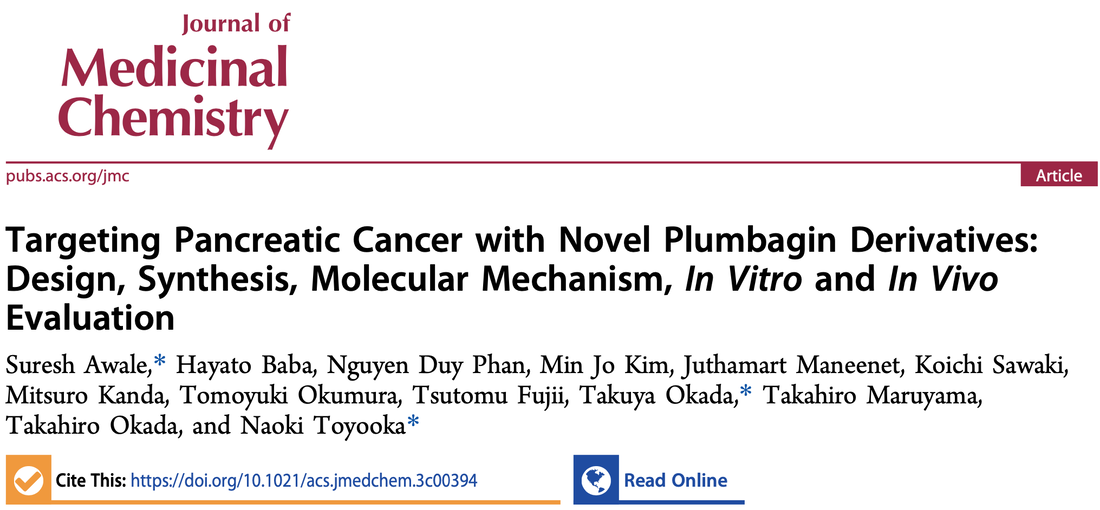
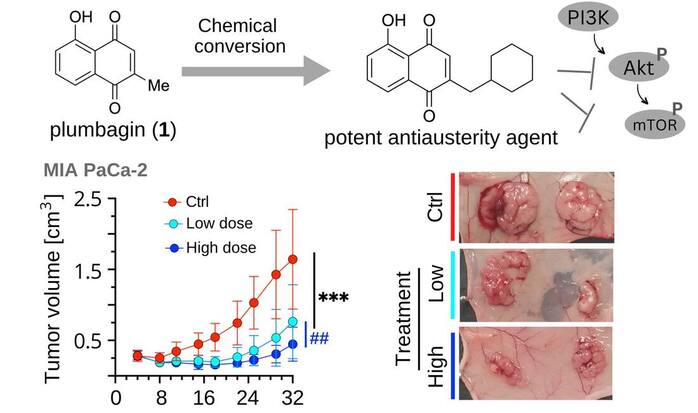
Our Natural Drug Discovery Lab is dedicated to the development of new drugs for pancreatic cancer, one of the most deadly forms of cancer with a five-year survival rate of just five to ten percent. The incidence of pancreatic cancer has been steadily increasing by 1% annually since 2000 and is projected to be the second leading cause of cancer death by 2030. Currently, there are no effective drugs available for treating this cancer, which is particularly difficult to treat as it is often diagnosed at an advanced stage when it has already spread to other parts of the body.
Our lab is focused on investigating medicinal plants as a potential source of new drugs for pancreatic cancer. Our research team is actively working to discover new classes of drugs that have the potential to revolutionize the treatment of pancreatic cancer.One of the unique aspects of our research is our focus on targeting the "austerity" phenomenon in cancer. This refers to a shift in energy metabolism and the activation of specific enzymes and pathways that allow cancer cells to generate energy and other necessary biomolecules under conditions of nutrient deprivation. Pancreatic cancer cells, which grow in conditions of low nutrient and oxygen supply due to hypovascularity, may upregulate alternative energy pathways, such as the pentose phosphate pathway or fatty acid oxidation, to generate energy from other sources and survive in the nutrient-deprived tumor microenvironment. Our lab has discovered a number of unique anti-cancer agents that target the PI3/Akt/mTOR and autophagy pathways and selectively kill pancreatic cancer cells without affecting normal cells. This is a significant advancement in the field as current chemotherapy drugs have significant limitations, including toxic side effects and the development of resistance.
We believe that our research will have a significant impact in the fight against pancreatic cancer. With our focus on natural compounds and targeting the unique characteristics of pancreatic cancer, we are confident that we will be able to discover new and effective drugs for the treatment of this deadly disease. We are committed to continuing our research efforts and making advances in the field of pancreatic cancer treatment. Our lab invites collaboration with other researchers and pharmaceutical companies to accelerate the development of new drugs to combat pancreatic cancer. We invite you to follow our publications and research progress, to stay updated on the cutting-edge research and latest discoveries that we make in the fight against pancreatic cancer.
Our lab is focused on investigating medicinal plants as a potential source of new drugs for pancreatic cancer. Our research team is actively working to discover new classes of drugs that have the potential to revolutionize the treatment of pancreatic cancer.One of the unique aspects of our research is our focus on targeting the "austerity" phenomenon in cancer. This refers to a shift in energy metabolism and the activation of specific enzymes and pathways that allow cancer cells to generate energy and other necessary biomolecules under conditions of nutrient deprivation. Pancreatic cancer cells, which grow in conditions of low nutrient and oxygen supply due to hypovascularity, may upregulate alternative energy pathways, such as the pentose phosphate pathway or fatty acid oxidation, to generate energy from other sources and survive in the nutrient-deprived tumor microenvironment. Our lab has discovered a number of unique anti-cancer agents that target the PI3/Akt/mTOR and autophagy pathways and selectively kill pancreatic cancer cells without affecting normal cells. This is a significant advancement in the field as current chemotherapy drugs have significant limitations, including toxic side effects and the development of resistance.
We believe that our research will have a significant impact in the fight against pancreatic cancer. With our focus on natural compounds and targeting the unique characteristics of pancreatic cancer, we are confident that we will be able to discover new and effective drugs for the treatment of this deadly disease. We are committed to continuing our research efforts and making advances in the field of pancreatic cancer treatment. Our lab invites collaboration with other researchers and pharmaceutical companies to accelerate the development of new drugs to combat pancreatic cancer. We invite you to follow our publications and research progress, to stay updated on the cutting-edge research and latest discoveries that we make in the fight against pancreatic cancer.
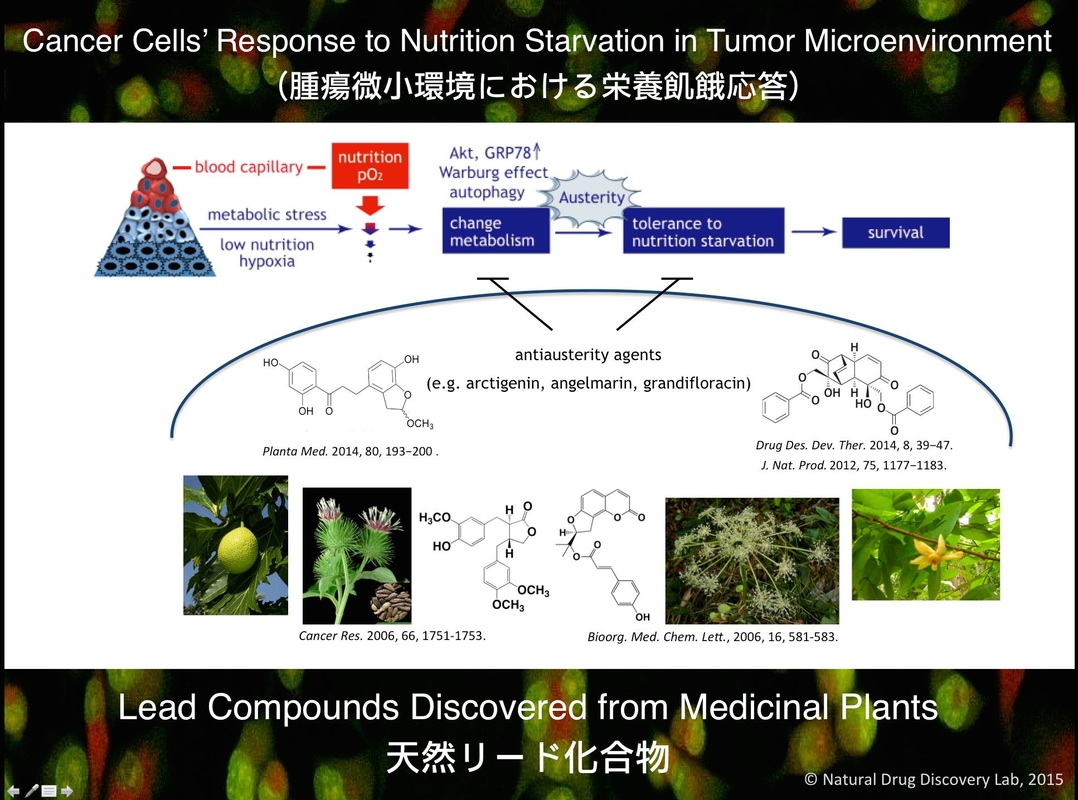
Natural Drug Discovery LabThe major research focus of our division is to discover natural anticancer agents by targeting cancer cells’ characteristic performance (e.g. tolerance to nutrient starvation) in the tumor microenvironment. Tumor cells in general proliferate very fast in an unregulated manner and are often exposed to nutrition and oxygen deficient environment due to poor and disorganized vasculature. In particular, cells of the clinically hypo-vascular tumors such as pancreatic cancers have inherent ability to tolerate the extreme environments of low nutrition and oxygen by modulating their energy metabolism (austerity). Therefore, to discover natural anticancer agents that retard this tolerance to nutrition starvation is one of the major research goals of our division. In order to achieve this goal, we are actively engaging in the following research projects.
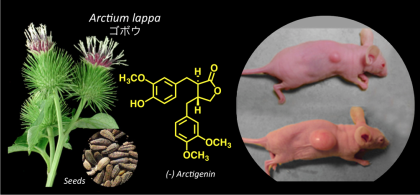
Arctigenin, an anti-cancer agent discovered through anti-austerity strategy showed potent anticancer activity against a number of human pancreatic xenograft tumor models.
Most importantly, arctigenin had recently completed Phase II human clinical trial against gemcitabine-refractory advanced pancreatic cancer patients. It showed a high safety profile and significant survival benefit among the pancreatic cancer patients. |
天然薬物開発研究室 我々 の分野では、研究の主な焦点として、腫瘍微小環境におけるがん細胞の栄養飢餓耐性を標的とする天然抗がん物質を探索している。がん細胞は一般的に無秩序か つ急速に増殖し、多くの場合、脆弱で無秩序に腫瘍血管が形成されるため栄養や酸素の欠如した環境に曝されることになる。特に、膵臓がんのような乏血性腫瘍 においては、がん細胞はエネルギー代謝を調節することで低栄養・低酸素といった厳しい環境に対する耐性(austerity)を獲得している。従って、この栄養飢餓に対する耐性を解除する天然抗がん化合物の探索は、我々の重要な研究目標の1つである。この研究目標を達成するため、我々の分野では以下の研究課題に積極的に取り組んでいる。
|
My outlook and intentions are holistic, in that I believe disciplines have much to offer each other: I am passionate about bridging the traditional medical knowledge within chemistry and biology; I remain convinced that assiduous effort with guarded optimism will yield practical, important outcomes. My mantra is “to search for possibilities where there seem to be but impossibilities” and to advance Traditional Natural Medicine research and its attendant drug discoveries in contributing to improved health in our global society.
Suresh Awale
私たちの研究世界ニュースでClick here to see Our Research in the World News Outlet
Press Release by American Chemical Society
FOR IMMEDIATE RELEASE, ACS News Service: November 14, 2018
American Chemical Society announced a press release (Nov. 14, 2018) featuring our latest research published in the Journal of Natural Products under the title "Rainforest vine compound starves pancreatic cancer cells.” The press release has been disseminated to the thousands of journalists, ACS members, and to the public around the world.
Click here for the press release link
Click here for the press release link
Suresh Awale准教授らの研究がACS(アメリカ化学会)の注目論文に選定されました
本学和漢医薬学総合研究所のSuresh Awale 准教授らがドイツのヴュルツブルク大学有機化学研究所のGerhard Bringmann (ホームページ)教授らと共著で発表した論文は2018年11月14日付のAmerican Chemical Society(アメリカ化学会)PressPac に選ばれ、ACSのホームページに掲載されました。ACS編集部が特別記事として本論文の概要をタイトル「Rainforest vine compound starves pancreatic cancer cells」の下に紹介し、全世界何千もの報道関係者及びACSメンバー全員に配信しています。
論文名:Ancistrolikokine E3, a 5,8′-Coupled Naphthylisoquinoline Alkaloid, Eliminates the Tolerance of Cancer Cells to Nutrition Starvation by Inhibition of the Akt/mTOR/Autophagy Signaling Pathway (DOI: 10.1021/acs.jnatprod.8b00733)
著者: Suresh Awale,* Dya Fita Dibwe, Chandrasekar Balachandran, Shaimaa Fayez, Doris Feineis, Blaise Kimbadi Lombe, and Gerhard Bringmann*
本論文はアフリカの植物から強い抗膵臓がん作用を有する新規化合物の発見及びその作用のメカニズムを発表しました。
詳細は下記よりご確認頂けます。
本学和漢医薬学総合研究所のSuresh Awale 准教授らがドイツのヴュルツブルク大学有機化学研究所のGerhard Bringmann (ホームページ)教授らと共著で発表した論文は2018年11月14日付のAmerican Chemical Society(アメリカ化学会)PressPac に選ばれ、ACSのホームページに掲載されました。ACS編集部が特別記事として本論文の概要をタイトル「Rainforest vine compound starves pancreatic cancer cells」の下に紹介し、全世界何千もの報道関係者及びACSメンバー全員に配信しています。
論文名:Ancistrolikokine E3, a 5,8′-Coupled Naphthylisoquinoline Alkaloid, Eliminates the Tolerance of Cancer Cells to Nutrition Starvation by Inhibition of the Akt/mTOR/Autophagy Signaling Pathway (DOI: 10.1021/acs.jnatprod.8b00733)
著者: Suresh Awale,* Dya Fita Dibwe, Chandrasekar Balachandran, Shaimaa Fayez, Doris Feineis, Blaise Kimbadi Lombe, and Gerhard Bringmann*
本論文はアフリカの植物から強い抗膵臓がん作用を有する新規化合物の発見及びその作用のメカニズムを発表しました。
詳細は下記よりご確認頂けます。
Copyright © 2023, Natural Drug Discovery Lab, All Rights Reserved
|
|